What is acetic acid? Properties of Acetic & Uses of Acetic
We associate acetic acid generally with vinegar. The sour and pungent smell you smell in vinegar is due to acetic acid only. This acid is well-known mostly because of its strong presence in vinegar.
However, this acid has many other uses as well. It is essentially a household item which comes in use a lot of times daily.
What is acetic acid?
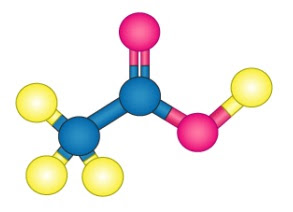
Acetic acid is also known as ethanoic acid, ethylic acid, vinegar acid, and methane carboxylic acid; it has the chemical formula of CH3COOH. Acetic acid is a byproduct of fermentation, and gives vinegar its characteristic odor. Vinegar is about 4-6% acetic acid in water. More concentrated solutions can be found in laboratory use, and pure acetic acid containing only traces of water is known as glacial acetic acid.
Acetic acid is the 33rd highest volume chemical produced in the United States. Acetic acid is used in the manufacture of acetic anhydride, cellulose acetate, vinyl acetate monomer, acetic esters, chloracetic acid, plastics, dyes, insecticides, photographic chemicals, and rubber. Other commercial uses include the manufacture of vitamins, antibiotics, hormones, and organic chemicals, and as a food additive. Typical concentrations of acetic acid occurring naturally in foods are 700 to 1,200 milligrams/kilogram (mg/kg) in wines, up to 860 mg/kg in aged cheeses, and 2.8 mg/kg in fresh orange juice.
Uses of Acetic Acid
Ethanoic acid is a very important organic compound in the day-to-day lives of humans. Some important uses of acetic acid are listed below.
Acetic acid is used as an antiseptic due to its antibacterial qualities
The manufacture of rayon fibre involves the use of ethanoic acid.
Medically, acetic acid has been employed to treat cancer by its direct injection into the tumour.
Being the major constituent of vinegar, it finds use in the pickling of many vegetables.
The manufacture of rubber involves the use of ethanoic acid. It is also used in the manufacture of various perfumes.
It is widely used in the production of VAM (vinyl acetate monomer).
When two molecules of acetic acid undergo a condensation reaction together, the product formed is acetic anhydride.
Physical Properties of Acetic Acid
Some important physical properties of acetic acid are listed below.
Ethanoic or acetic acid has a pungent vinegar odour and sour taste.
It is a colourless liquid.
It boils at 391K.
Its density in liquid form is 1.049 g/cm³.
It can mix with water, alcohol, ether in all proportions.
In water, it dissolves with the evolution of heat and contraction in volume.
It is corrosive in nature and produces blisters when in contact with the skin.
Sulphur, iodine, and many other organic compounds are dissolved in it.
Chemical Properties of Acetic Acid
Some important chemical properties of acetic acid are given below:
The carboxyl functional group in ethanoic acid causes ionization of the compound, given by the reaction: CH₃COOH ⇌ CH₃COO⁻ + H⁺
The acidic quality of acetic acid comes from the release of the proton, described by the equilibrium reaction above.
In a solution of water, the acid dissociation constant (pKa) of ethanoic acid 4.76.
CH₃COO⁻, acetate is the conjugate base of acetic acid.
Acetic acid doesn't dissociate completely as it can be seen that the pH of an ethanoic acid solution of 1.0M concentration is 2.4.
Acetic acid is a polar, protic solvent, with a dielectric constant of 6.2 in its liquid form.
Frequently Asked Questions-FAQs
Is acetic acid the same as vinegar?
What is the difference between Acetic Acid and Vinegar? Vinegar contains acetic acid and water. Therefore, somewhat diluted acetic acid is found in vinegar. Other than acetic acid, natural vinegar may contain other compounds like citric acid, tartaric acid, etc.
What foods contain acetic acid?
Less commonly known as ethanoic acid, acetic acid is the primary ingredient in vinegar. This colourless acid has a pungent sour smell. There are many different types of vinegars, including apple cider vinegar, red and white wine vinegar, balsamic vinegar, malt vinegar, and rice vinegar.
Is acetic acid safe to consume?
POLICY: Acetic acid is generally recognized as safe for use in foods if it is of "food-grade" and is used in accord with good manufacturing processes. Acetic acid is considered "food-grade" if it complies with the specifications in Food Chemicals Codex. Diluted acetic acid is not vinegar.
Is acetic acid good for skin?
Acetic acid has antibacterial and antifungal properties. These properties could help clean the skin and prevent infections from bacteria or fungi.
Which is stronger acetic acid or vinegar?
White vinegar tends to have seven percent acetic acid, which is a higher level than other vinegars. Slightly milder vinegars, such as balsamic and red wine vinegar, have about six percent, and a relatively mild rice wine vinegar is around four and a half percent (none of which you would use for ricotta).
Is acetic acid cancerous?
It is unknown whether acetic acid could cause cancer in humans, but studies in animals and cell lines show no link to cancer or birth defects.
Acetic acid side effects
Get emergency medical help if you have any of these signs of an allergic reaction: hives; difficult breathing; swelling of your face, lips, tongue, or throat. Stop using acetic acid and call your doctor at once if you have severe burning or other irritation after using the ear drops.
Can we use lemon juice instead of vinegar?
The simple answer is yes, you may use lemon (or lime juice) in place of vinegar in home canning recipes, as lemon and lime juice are slightly more acidic than vinegar. Some people prefer the tastes of lemon or lime juice over vinegar, as they feel it has a milder flavor.
What is acetic acid made of?
Most acetic acid is made by methanol carbonylation, where methanol and carbon monoxide react to produce acetic acid. The compound is miscible with ethanol, ethyl ether, acetone, and benzene, and is soluble in carbon tetrachloride and carbon disulfide.
Comments
Post a Comment